Case of the week
A 54 years old, male patient presented with complaints of swelling in the bilateral lower limbs and breathlessness.
A 54 years old, male patient presented with complaints of swelling in the bilateral lower limbs and breathlessness.
View Case37 year old male presented with sudden loss of consciousness following nocturnal micturition with abnormal screening chest X- ray.
37 year old male presented with sudden loss of consciousness following nocturnal micturition with abnormal screening chest X- ray.
View CaseA 27 year old gentleman presented with few episodes of hemoptysis. He also had a history of cough with expectoration since few months.
A 27 year old gentleman presented with few episodes of hemoptysis. He also had a history of cough with expectoration since few months.
View CaseWalk in OPD patient with History of on and off mild breathlessness and fever since 6 months.
Walk in OPD patient with History of on and off mild breathlessness and fever since 6 months. No history of tuberculosis or ICU admission.
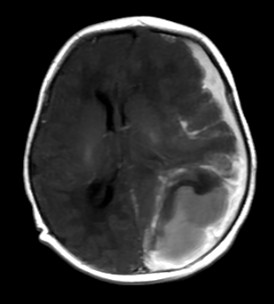
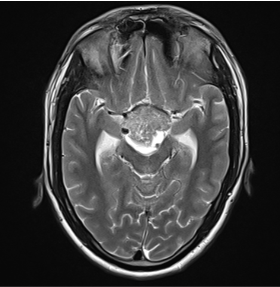
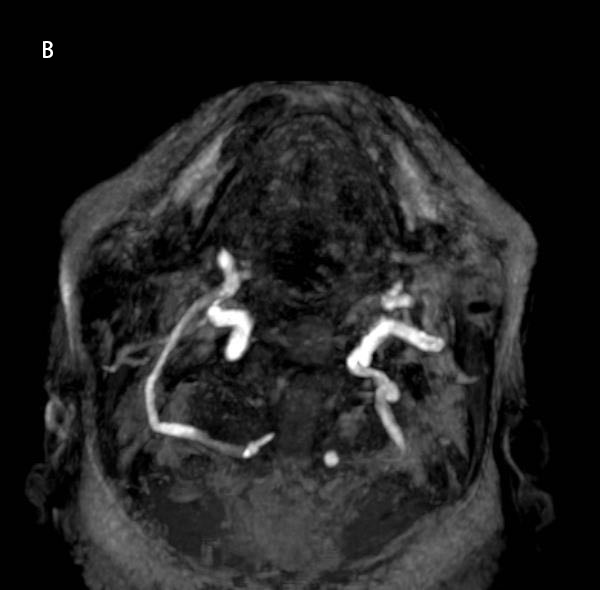
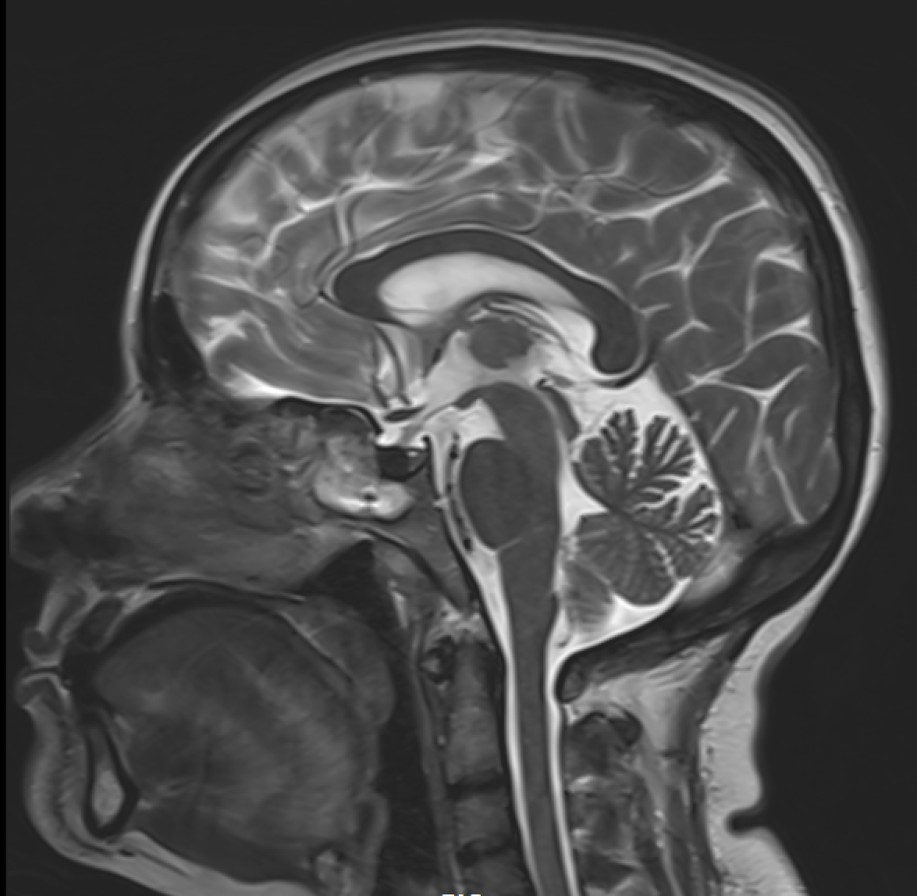
View Case5 days old infant presented with history of seizure. History of prolonged difficult delivery present.
5 days old infant presented with history of seizure. History of prolonged difficult delivery present.
View CaseA 18 year old male, History of headache since 1 month. Change of voice since 1 month.
A 18 year old male, History of headache since 1 month.Change of voice since 1 month.
View Case75y old male presented with numbness in right upper limb since 1 day associated with slurring of speech
75y old male presented with numbness in right upper limb since 1 day associated with slurring of speech
View CaseA 43-year-old lady who was a known case of chronic kidney disease was being evaluated for headache and orbito - nasal symptoms
A 43-year-old lady, who was a known case of chronic kidney disease was being evaluated for headache and orbito - nasal symptoms
View Case