A 43-year-old lady who was a known case of chronic kidney disease was being evaluated for headache and orbito - nasal symptoms
Clinical Details
- A 43-year-old lady, who was a known case of chronic kidney disease was being evaluated for headache and orbito - nasal symptoms.
- She had been undergoing dialysis for 3 years.
- Further evaluation with Contrast Enhanced MRI Brain with screening of paranasal sinuses and orbits was suggested.
Imaging:
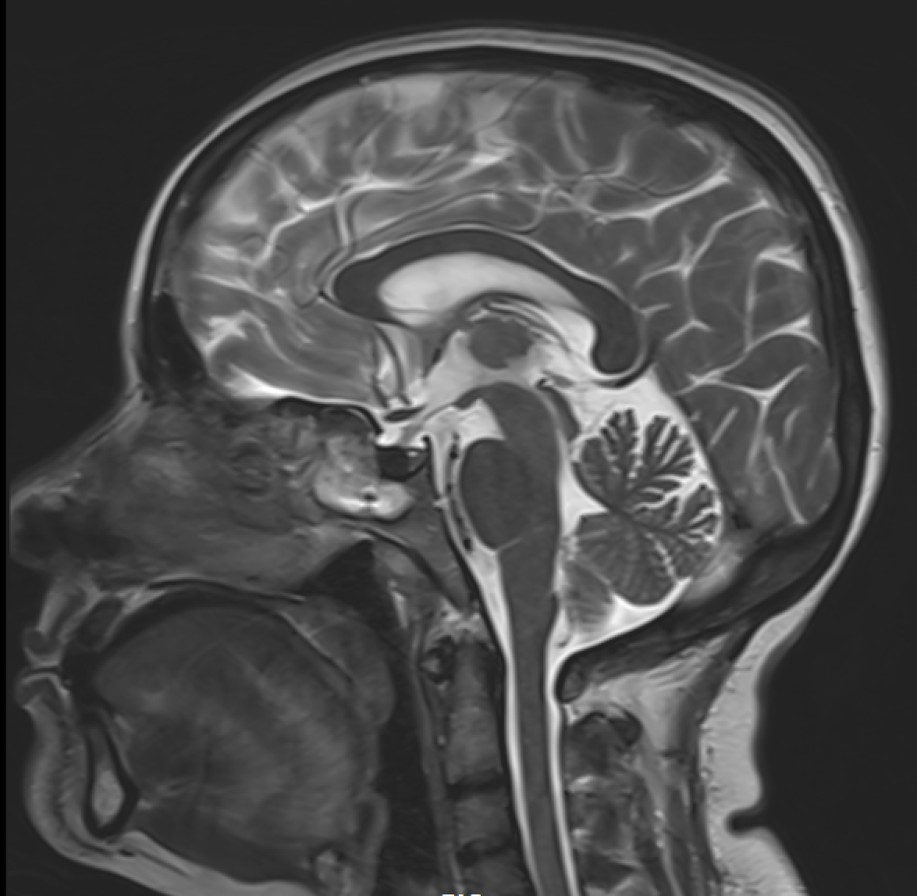
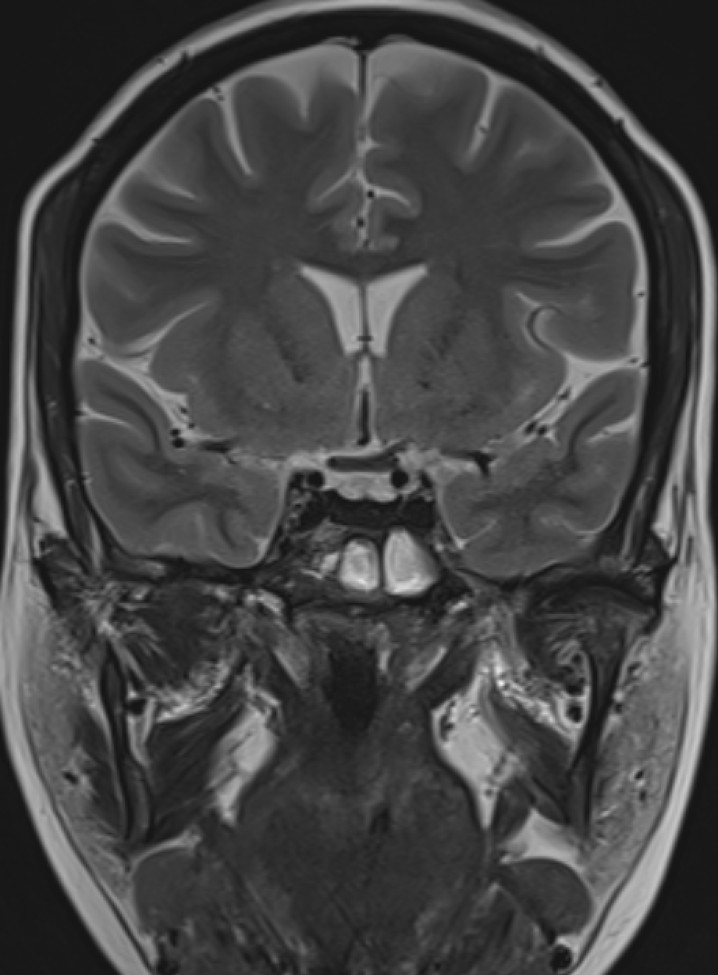
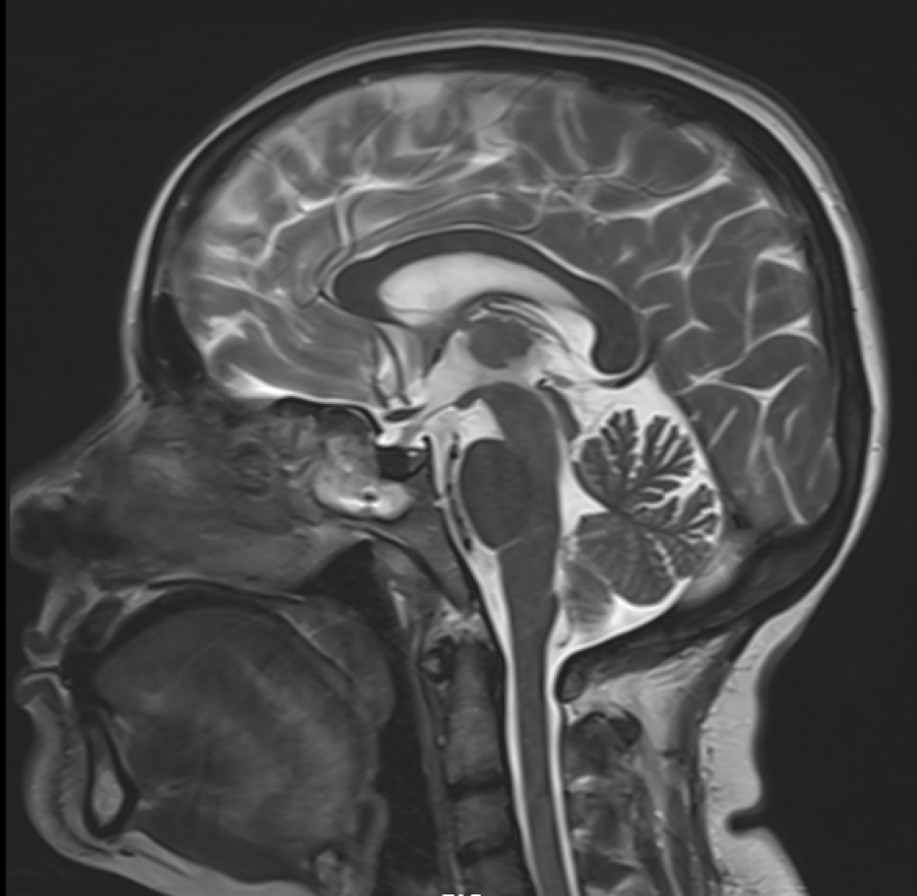
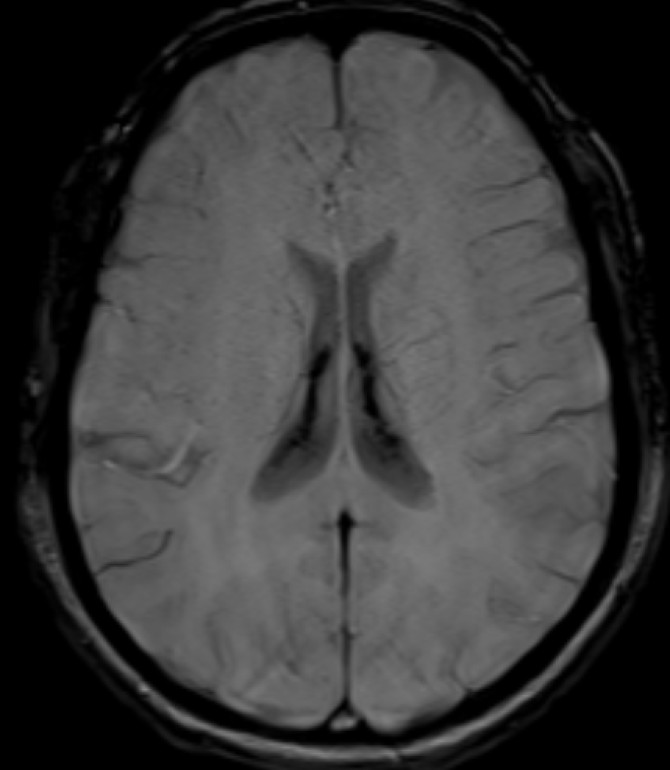
- T2 weighted midsagittal and coronal images of the brain.
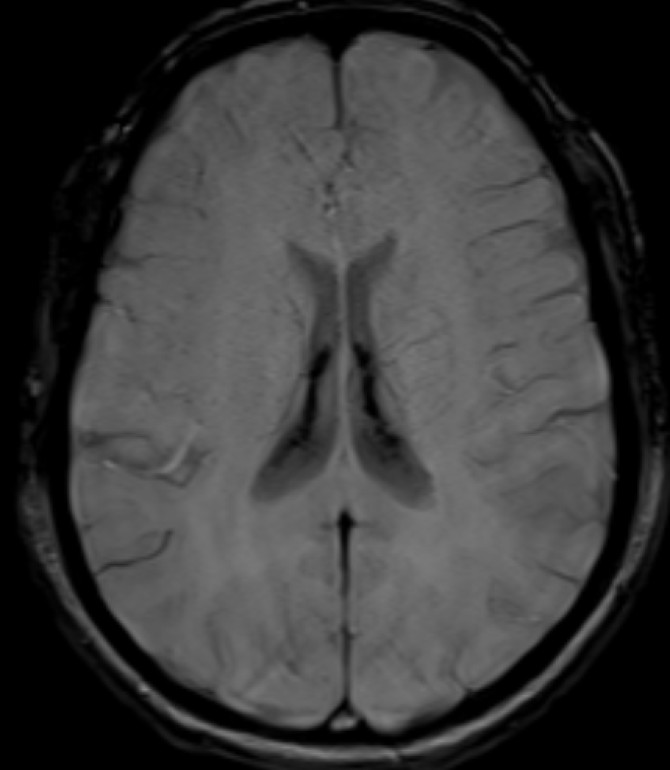
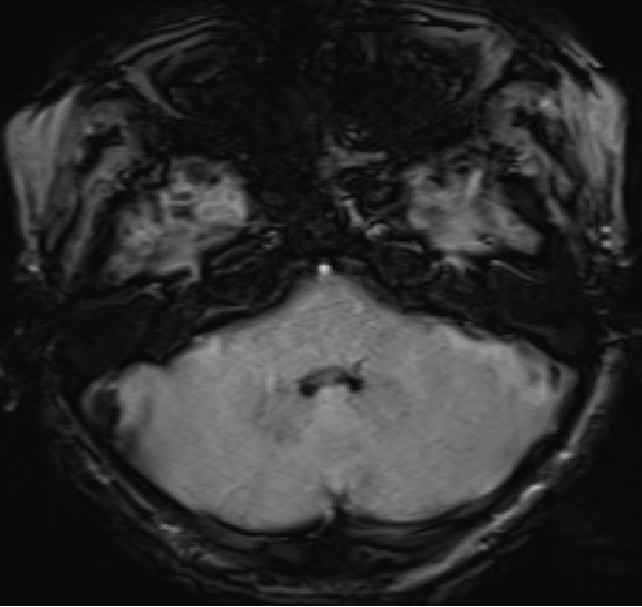
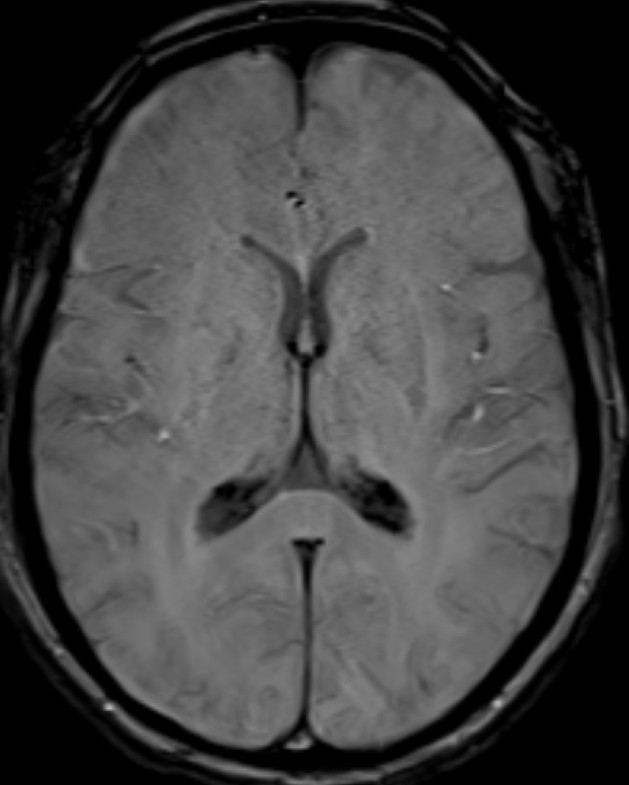
- Axial SWI images of the brain at the level of body and atrium of lateral ventricles.
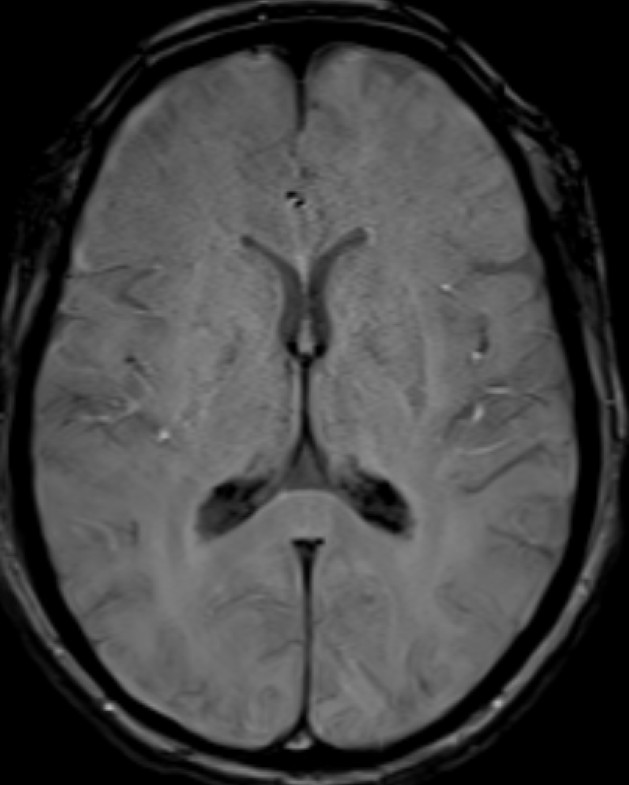
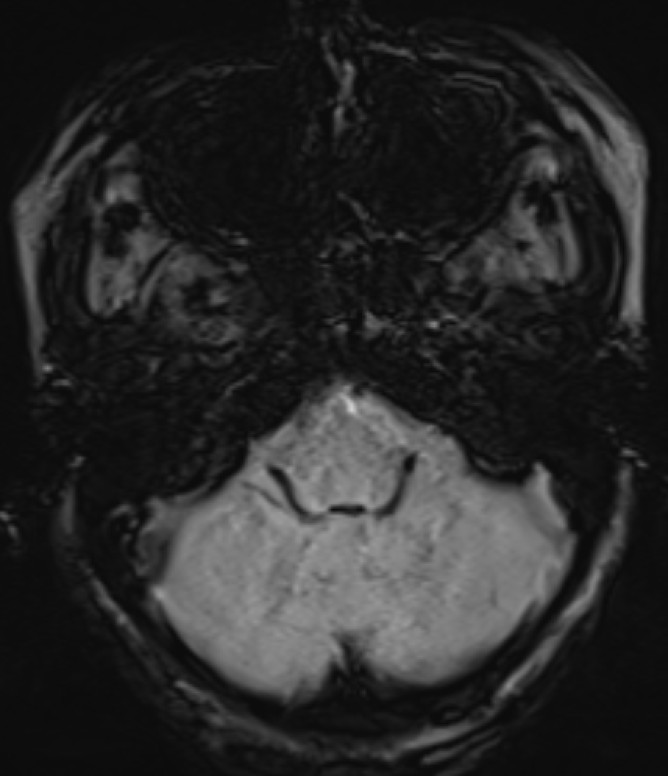
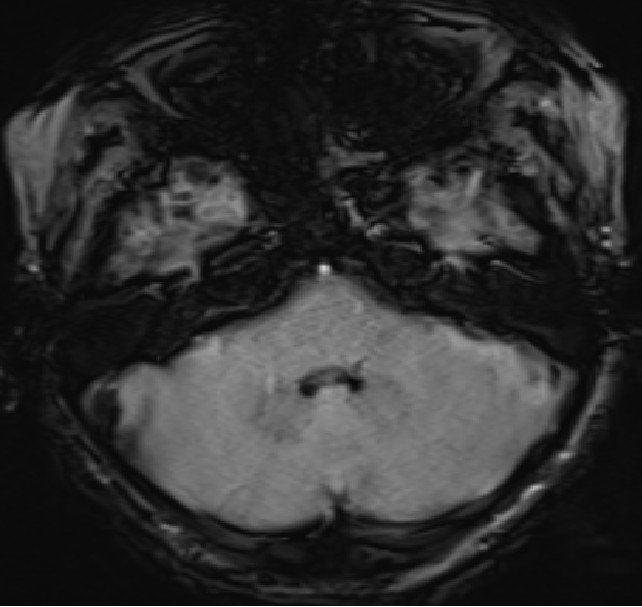
- Axial SWI images of the brain at the level of the 4th ventricle and foramina of Luschka.
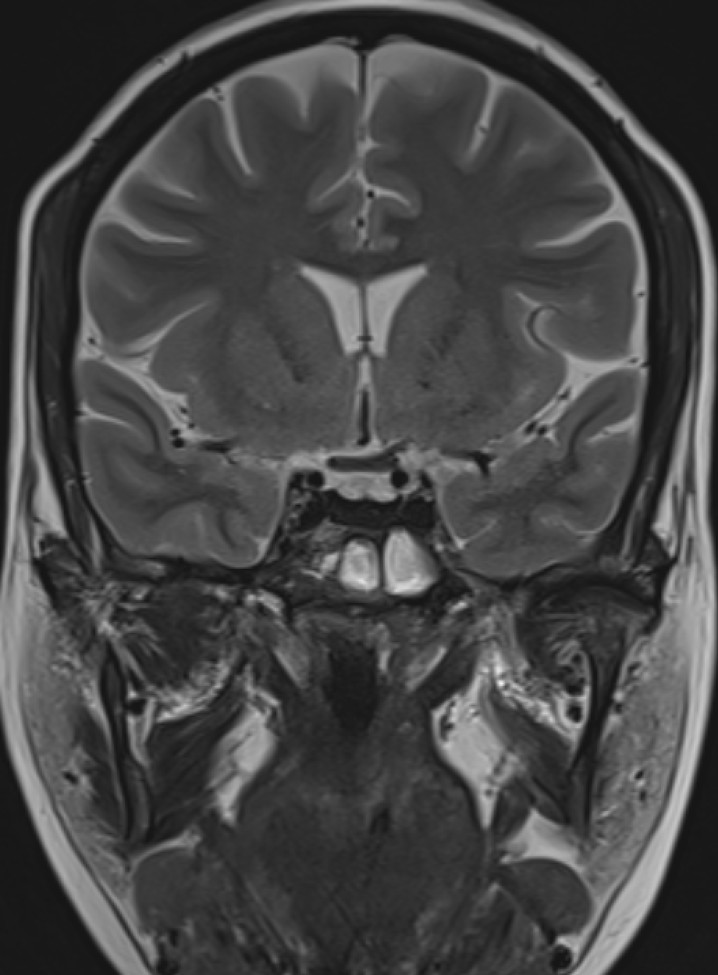
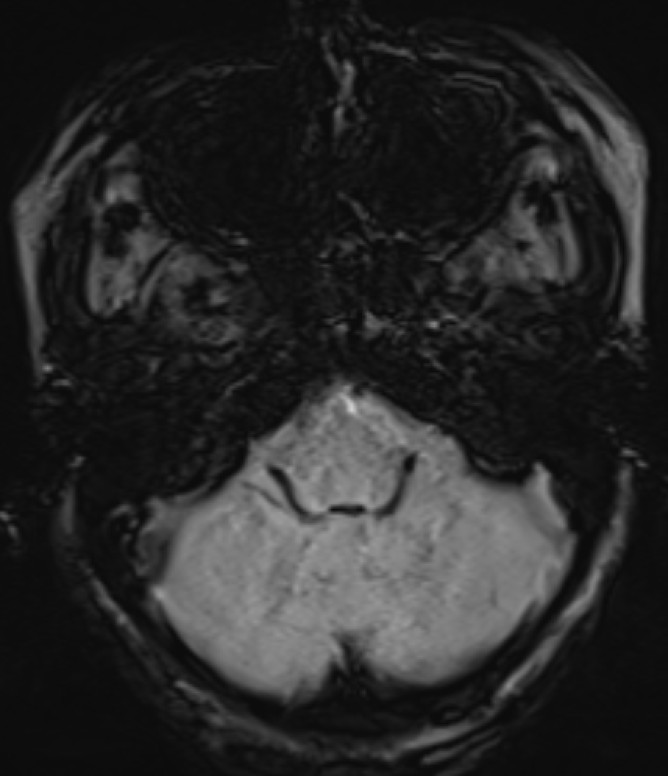
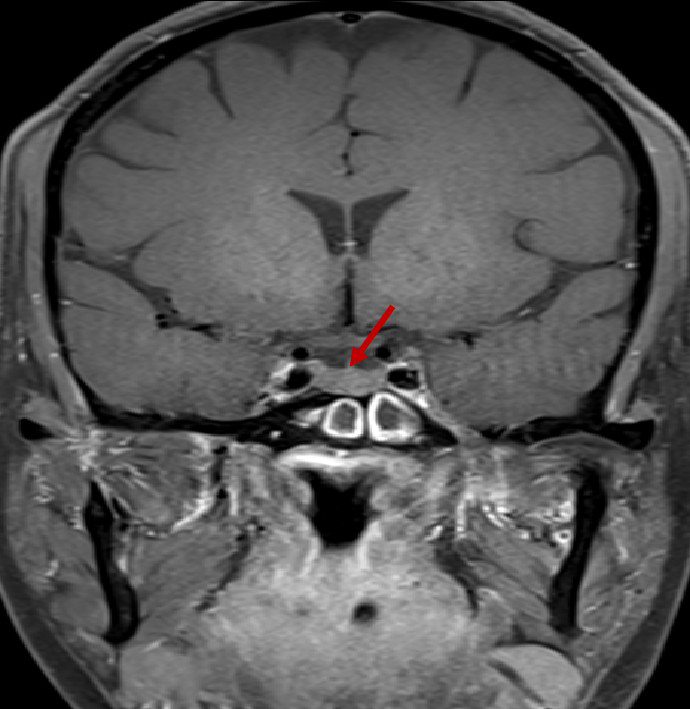
- Post contrast T1FS coronal image of the brain.
- Diffuse T2 hypo intensity of the pituitary gland. (red arrow)
- Booming seen diffusely along the choroid plexus in the lateral and 4th ventricles. (red arrows)
- Diffusely reduced post contrast enhancement of the pituitary gland. (red arrow)
- Blooming seen diffusely along the choroid plexus in the lateral ventricles. (red arrows)
- Blooming seen diffusely along the choroid plexus in the 4th ventricle and foramina of Lushkae. (red arrows)
Diagnosis:
- Incidentally noted imaging manifestations of CNS haemochromatosis involving the pituitary gland and choroid plexus.
Discussion:
Introduction
- Iron overload and deposition in organs (hemochromatosis) can be classified as primary or secondary. Primary hemochromatosis is a recessive autosomal genetic disorder that alters a protein involved in the regulation of iron absorption with resultant increased iron.
- Any other nongenetic cause of iron accumulation in the organs is classified as secondary hemochromatosis. The causes of secondary hemochromatosis include (a) another cause of increased absorption, such as cirrhosis, (b) myelodysplastic syndrome, (c) anemias related to ineffective erythropoiesis (eg, thalassemia), and (d) exogenous increase by ingestion, parenteral infusion, or multiple transfusions.
- In patients with chronic kidney disease, concurrent anemia is common and hemosiderosis is known to occur due to iatrogenic causes (parenteral iron, EPO, dialysis, transfusion). Hemochromatosis has seldom been described.
Pathophysiology
- Excess iron is deposited in the parenchymal cells of various organs, especially in the heart, liver - spleen (reticuloendohelial system), bones - joints, skin, and endocrine glands, and eventually leads to cellular damage and organic dysfunction.
- When these organs get saturated, deposition occurs in other tissues.
- The brain cells are normally well shielded from pathologic iron accumulation due to the blood brain barrier (BBB). Nevertheless, some CNS structures lay outside the BBB in which iron deposition can occur, such as the pituitary gland, the choroid plexus, and the circumventricular organs.
- The choroid plexus is a highly vascularized papillary structure that protrudes into the ventricles. Capillaries of the choroid plexus are fenestrated, non-continuous, and permeable to small molecules and water. However, adjacent choroidal epithelial cells form tight junctions that selectively prevent the passage of ions and macromolecules. According to the autoradiographic distribution of Fe, iron is selectively taken up by choroid plexus and extra-blood-brain barriers from the blood. Choroid plexus is hypothesized to play a role in protecting the brain from iron overloading through this buffering mechanism. (hence, the presence of choroid plexus hemosiderosis can be considered as an early signal of CNS iron deposition)
- In the anterior pituitary gonadotrophic cells seem to be most susceptible to iron deposition, which explains the association between hemochromatosis and hypogonadotropic hypogonadism.
Clinical Profile
- In the early stages, hemochromatosis is asymptomatic.
- Iron deposition in the liver and heart can lead to chronic liver parenchyma disease - cirrhosis and cardiac failure respectively. Cardiomyopathy may occur.
- CNS hemosiderosis is usually asymptomatic, but if not diagnosed early, it can cause pituitary dysfunction (especially hypogonadotropic hypogonadism/ hypopituitarism) or neurologic sequelae (movement disorders/ focal deficit).
- Iron overload (hemosiderosis) is evaluated using serum transferrin saturation and serum ferritin concentration levels. Biopsy is considered the gold standard for evaluation of haemochromatosis, however, it is invasive. Magnetic resonance imaging, especially T2* imaging is a useful non – invasive adjunct for the evaluation of haemochromatosis.
Imaging
- Iron deposition is typically observed at sites outside the blood-brain barrier, which include the pituitary gland, choroid plexus (MRI choroid plexus sign of haemochromatosis), pineal gland, and area postrema (circumventricular organs) as hypointense signal on T2, GRE and SWI images.
- MRI, especially T2*-weighted imaging/ GRE, effectively detects iron overloading because of a striking reduction of T2 relaxation time.
- Susceptibility weighted imaging combines magnitude and phase information to accentuate the visibility of susceptible foci and is more sensitive than conventional GRE sequences for detecting microhemorrhages, subarachnoid hemorrhage, deoxygenated blood, blood degradation products.
Treatment
- Treatment of haemochromatosis generally includes phlebotomy and chelation therapy.
- Phlebotomy, which removes excessive iron and maintains normal iron storage, remains the cornerstone therapy for hereditary haemochromatosis.
- In patients with haemochromatosis and heart disease, anaemia or poor venous access, treatments with iron chelation agents are recommended.
- In ESRD patients with secondary haemochromatosis, chelation treatment should be combined with haemodialysis.
Conclusion
- CNS haemochromatosis is often clinically silent but can be diagnosed with imaging methods; therefore, radiologists should be aware of the MR imaging findings so that they can suggest the diagnosis.
- Moreover, the evaluation of the effects of clinical treatment can also be done in a noninvasive way.
- Early diagnosis can lead to timely treatment before irreversible organ damage develops.
References
- Verberckmoes B, Dekeyzer S, Decaestecker K. The Dark Pituitary: Hemochromatosis as a Lesser-Known Cause of Pituitary Dysfunction. J Belg Soc Radiol. 2022 Jun 29;106(1):63. doi: 10.5334/jbsr.2771.
- Kim MS, Lee HY, Lim MK, Kang YH, Kim JH, Lee KH. Transfusional Iron Overload and Choroid Plexus Hemosiderosis in a Pediatric Patient: Brain Magnetic Resonance Imaging Findings. Investig Magn Reson Imaging. 2019 Dec;23(4):390-394. https://doi.org/10.13104/imri.2019.23.4.390
- https://pubs.rsna.org/doi/abs/10.1148/rg.296095511
- Kira R, Ohga S, Takada H, Gondo K, Mihara F, Hara T. MR choroid plexus sign of iron overload. Neurology 2000;55:1340.
Dr Rahul Karthik Lingutla
Consultant Radiologist
MHRG, Manipal Hospital, Yeshwanthpur
Dr Kanupriya Grover
Cross Sectional Imaging Fellow
MHRG, Manipal Hospital, Yeshwanthpur