4 month old infant with antenatally detected congenital hear disease, Now presenting with breathlessness
4-month-old infant with antenatally detected congenital heart disease, Now presenting with breathlessness
FINDINGS
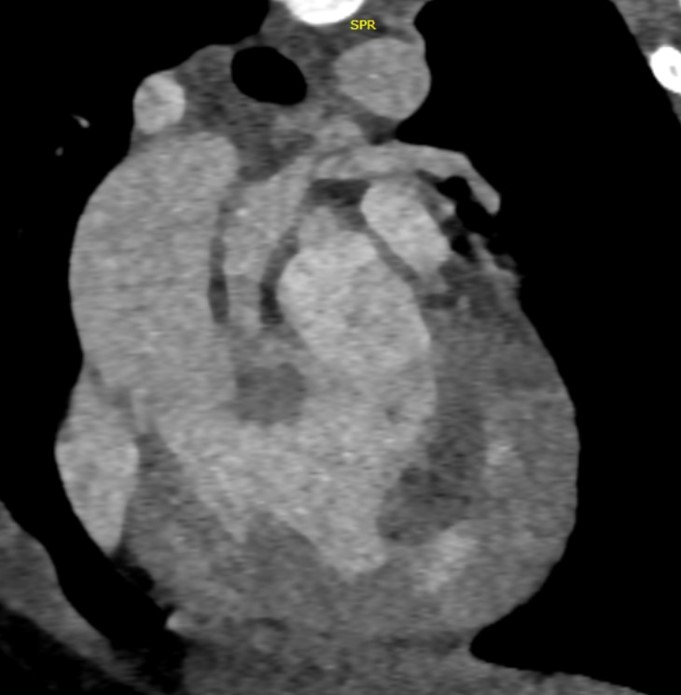
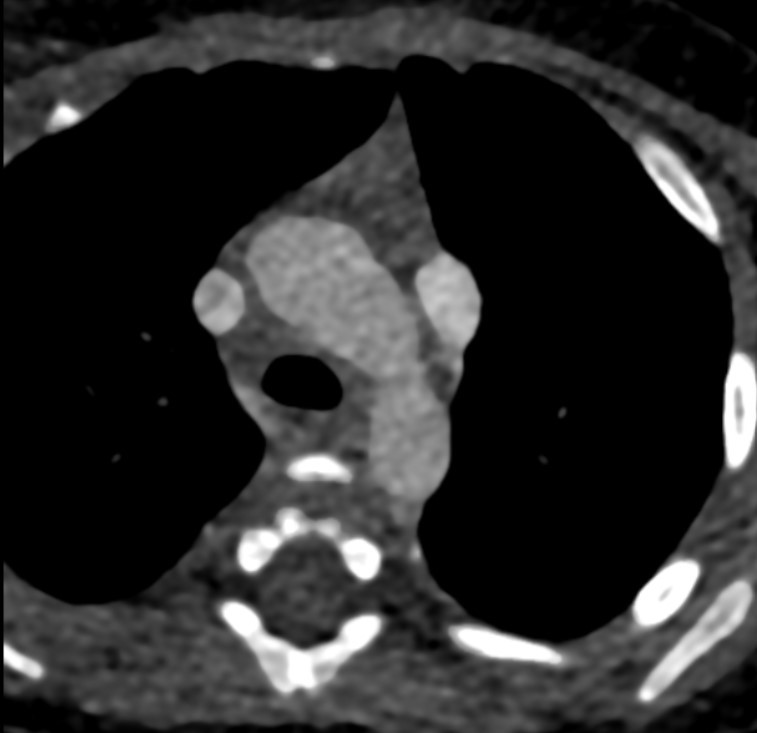
- Cardiac CTA in axial sections showing double SVC (a) on left side at level of trachea draining into common atrial cavity.
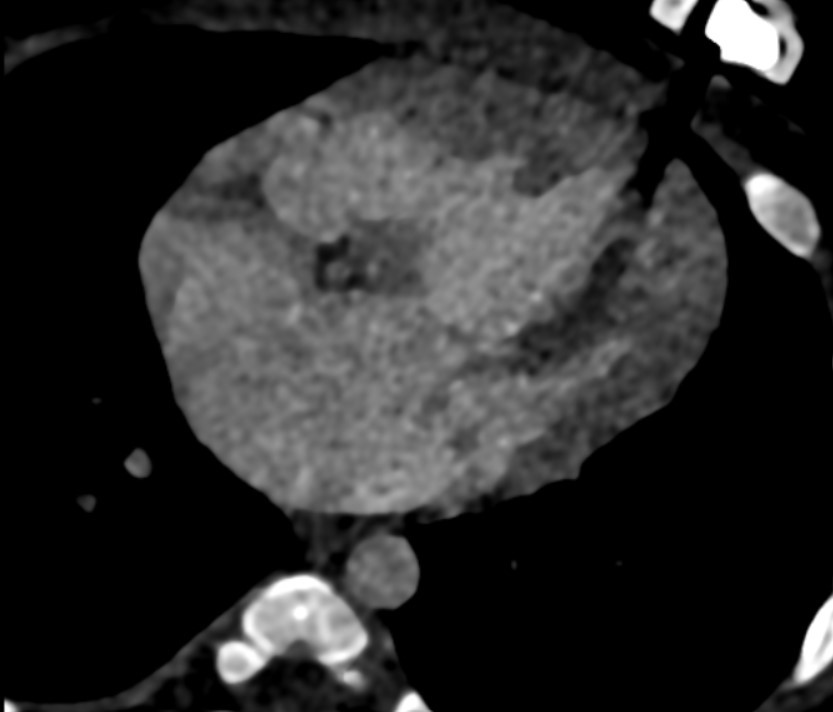
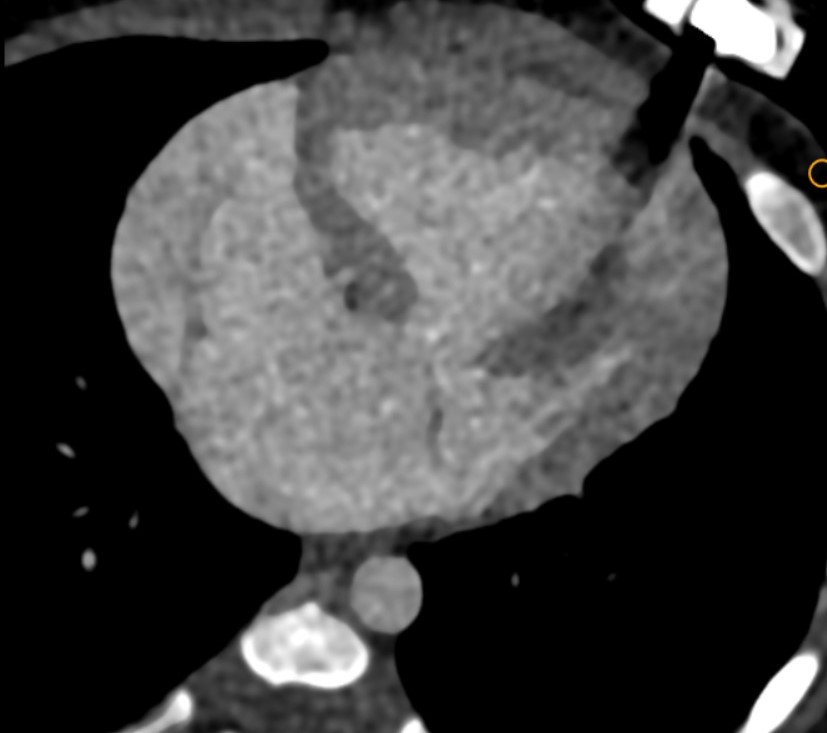
At the level of cardia, large common atrial cavity (b), AV defect (c), large VSD (d), dilated and hypertrophic right ventricle (e) and hypoplastic left ventricle (f). - Cardiac CTA in axial section showing large common atrial cavity and aortic root (j) communicating with dilated right ventricle ( i).
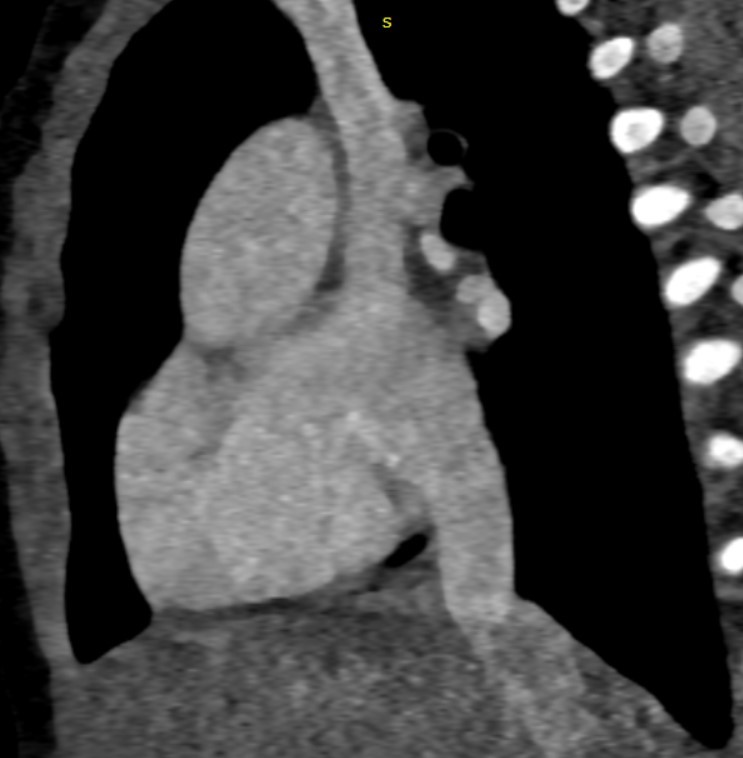
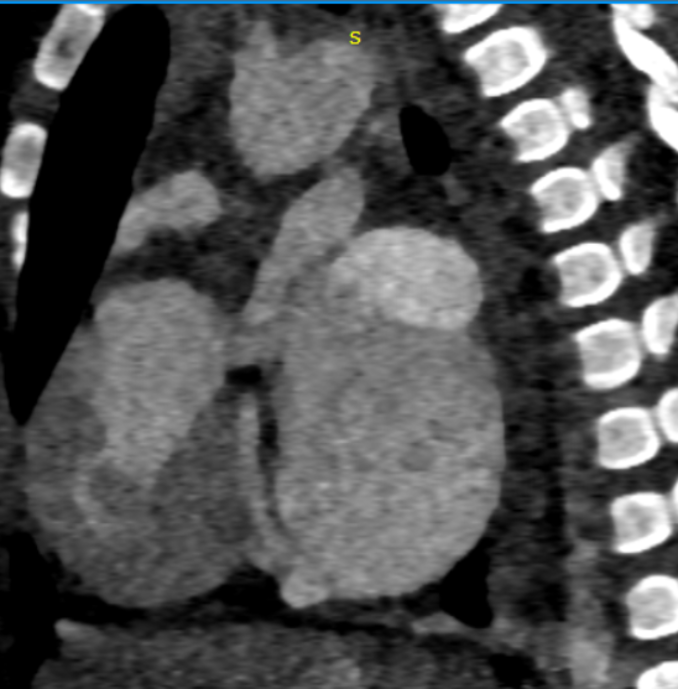
Sagittal oblique sections showing both SVC (g) and IVC (h) opening into common atrial cavity. - Cardiac CTA in coronal oblique sections showing Aorta (i) and main pulmonary artery (k)seen to arise from right ventricle.
Small PDA is seen connecting the aorta to the left branch of pulmonary artery (arrow)
Diagnosis: Double outlet right ventricle (DORV)
- Double outlet right ventricle (DORV) is a congenital cardiac anomaly where both the aorta and pulmonary trunk arise from the morphologically right ventricle.
- It is usually classed as a conotruncal anomaly.
- There is almost always a concurrent ventricular septal defect (VSD)
Associations
Aneuploidy/chromosomal
- trisomy 13 and 18
Cardiovascular/pulmonary
- congenital pulmonary stenosis
- coarctation of the aorta
- right-sided aortic arch
- anomalous pulmonary venous return
The classification of DORV is primarily based on the location of VSD and the presence/absence of pulmonary stenosis (PS), with the most common subtypes of DORV being as follows:
(i)Tetrology of Fallot-type variant (subaortic VSD with PS).
(ii)Transposition of great arteries-type variant (sub pulmonary VSD without PS): Taussig-Bing anomaly.
(iii)VSD-type variant (subaortic VSD without PS).
(iv)Univentricular heart–type variant (DORV with mitral atresia, unbalanced atrioventricular canal, or presence of severe hypoplasia of one of the ventricular sinuses).
Approach to Diagnosis on CT Imaging
The major points of concern in the imaging assessment include determining,
- Great vessel relationship.
- Identification and categorization of conus.
- Assessing the location and routabilty of the VSD.
- Identification of associated anomalies, which can have an impact on the management.
Great vessel relationship:
- The aorta is posterior, inferior, and to the right of the pulmonary artery, or can be malposed with the aorta being directly anterior or posterior and to the left of the pulmonary artery.
- The aorta can also be dextroposed or levoposed.
- In fact, it is the rotation of the great arteries driven by the conal development that is responsible for the diverse spectrum of DORV.
Identification and categorization of conus:
- In the most commonly observed classic tetralogy of Fallot-type DORV, there is a near-normal length of the conus beneath the pulmonary valve and minimal conus beneath the aortic valve. This results in aortomitral discontinuity, and the pulmonary valve assumes an anterior and superior position.
- In the second most common DORV variant has the conus mostly under the aortic valve along with a small amount of conus under the pulmonary valve, which results in loss of pulmonary-mitral continuity. This form is physiologically similar to transposition of the great arteries.
- The length of conus determines the location of VSD relative to the tricuspid valve and semilunar valve with a longer conus increasing the distance between the VSD and semilunar valves.
Routabilty:
- A highly important surgical determinant is the routabilty of the VSD.
- The location of the VSD, as determined from the RV side (repair is performed from the RV side), depends on its relationship to the semilunar valve and the orientation of the outlet septum.
- If the outlet septum is fused to the left margin of the VSD, it is subaortic and, if the outlet septum is fused to the right margin of the VSD, it is sub pulmonary.
- If the distance between the VSD and the semilunar valves is more than the size of the aortic valve, it is termed as noncommitted VSD. However, even in patients with long conal length, the VSD may appear remote. In such patients, alignment of the VSD to either outflow tract should be mentioned.
- In subaortic VSD, repair is carried out by creating an intraventricular tunnel using a Gore-Tex patch as a baffle to divert blood from the VSD to the aorta.
- Arterial switch operation with creation of aorta to ventricular tunnel is preferred in subpulmonary VSD with right ventricular outflow obstruction
Other Associated Anomalies With Surgical Considerations
Sizing of Ventricles
- Adequate size of ventricles and atrioventricular valves determine whether biventricular repair can be undertaken in such patients.
- Adequate right ventricular volume should be present for intraventricular baffling, and this can be measured preoperatively if retrospective CT is performed.
- In patients with tricuspid or mitral atresia, if ventricular size is small, staged palliative repair—as for single-ventricle physiology—is performed.
Aortic Anomalies
- Aortic anomalies or variations that can be encountered include a right-sided aortic arch, presence of aberrant subclavian artery, anomalous origin of pulmonary artery from the ascending aorta, bovine arch, patent ductus arteriosus, arch hypoplasia, and coarctation of aorta.
- In the subgroup of patients with left ventricular outflow obstruction such as arch hypoplasia, the VSD to pulmonary artery baffle can be created during repair.
Pulmonary Arterial Anomalies
- Commonly associated pulmonary arterial anomalies include infundibular PS, supravalvular PS, and/or atresia of pulmonary arteries.
- During intraventricular repair, PS can be repaired either by resection of the septum or by using a conduit or a patch. Anomalous Systemic and Pulmonary Venous Drainage Associated venous drainage
- anomalies include persistent left superior vena cava, retro aortic left brachiocephalic vein, and partial (PAPVC) and total anomalous pulmonary venous drainage (TAPVC).
- PAPVC and TAPVC are commonly seen in the right and left atrial isomerism, respectively.
Coronary Arterial Anomalies
- Coronary artery anomalies include the separate origin of the left circumflex and left anterior descending coronary artery, dilated right coronary artery, origin of left anterior descending from right coronary artery, and origin of all coronary arteries from the same aortic sinus.
Conclusion
- Noninvasive multiplanar and multidimensional CT imaging is invaluable in both the preoperative and postoperative assessment of patients with DORV.
- Dual-source CT scanners have a high spatial and temporal resolution and utilize low-dose modulation techniques that limit the radiation dose.
- Because of the widespread availability of CT, as compared with MRI, especially in the low-income group countries, the use of current generation CT scanners utilizing low-dose technology seems a well-suited choice in the imaging evaluation of DORV.
REFERENCE:
- Vitiello R, McCrindle BW, Nykanen D, et al. Complications associated with pediatric cardiac catheterization. J Am Coll Cardiol. 1998;32:1433–1440.
- Tangcharoen T, Bell A, Hegde S, et al. Detection of coronary artery anomalies in infants and young children with congenital heart disease by using MR imaging. Radiology. 2011;259:240–247.
- Shi K, Yang ZG, Chen J, et al. Assessment of double outlet right ventricle associated with multiple malformations in pediatric patients using retrospective ECG-gated dual-source computed tomography. PLoS One. 2015;10:e0130987.
- Lacour-Gayet F. Intracardiac repair of double outlet right ventricle. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2008;11: 39–43.
- Chen SJ, Li YW, Wang JK, et al. Three-dimensional reconstruction of abnormal ventriculoarterial relationship by electron beam CT. J Comp Assist Tomo. 1998;22:560–568.
- Freedon R, Smallhorn J. Double outlet right ventricle. In: Freedon R, Benson L, Smallhorn J, eds. Neonatal Heart Disease, 1st ed. London: Springer Verlag; 1992:453–470.
- Frank L, Dillman JR, Parish V, et al. Cardiovascular MR imaging of conotruncal anomalies. Radiographics. 2010;30:1069–1094. 8. Sondheimer HM, Freedom RM, Olley PM. Double outlet right ventricle: clinical spectrum and prognosis. Am J Cardiol. 1977;39: 709–714.
Dr. DEEPTI H.V
Consultant Radiologist
Manipal Hospitals Radiology Group(MHRG)
Manipal Hospital, Bengaluru.
Dr. Srinivas P
Fellow in Radiology
Manipal Hospitals Radiology Group(MHRG)
Manipal Hospital, Bengaluru.